Microcapsules enhance the efficiency of genome editing
European researchers have discovered that polymer and hybrid silica-coated microcapsules can be used to make genome editing with CRISPR-Cas9 more efficient. By simplifying and increasing the efficiency of genome editing, it is predicted that the team’s work will eventually help cure previously irremediable inherited diseases such as Alzheimer’s, haemophilia and more.
As explained by study co-author Boris Fehse, from the University Medical Center Hamburg-Eppendorf, CRISPR-Cas9 is based on the immune system of bacteria, which ensures recognition and eradication of viruses (bacteriophages) if they try to infect them more than once. For this purpose, bacteria incorporate short sequences of the viral genome into their own genome (in the CRISPR region) and utilise them as a template to synthesise short complementary RNAs that recognise a phage genome using the key-lock principle. The process is similar to human antibodies recognising pathogens that try to infect a person repeatedly.
“CRISPR–Cas9 is a revolutionary genome-editing technology that has enormous potential for the treatment of genetic diseases,” the study authors wrote in the journal Nanomedicine: Nanotechnology, Biology and Medicine.
Unfortunately, gene-editing technologies such as CRISPR-Cas9, ZFNs and TALENs all share the same problem — a lack of safe and efficient delivery.
“These delivery methods are highly toxic,” said Alexander Timin, a lead author on the study from Tomsk Polytechnic University (TPU). “When they are used, some of the edited cells die. For example, when delivering biomaterial by electroporation, only 40–50% of cells survive.”
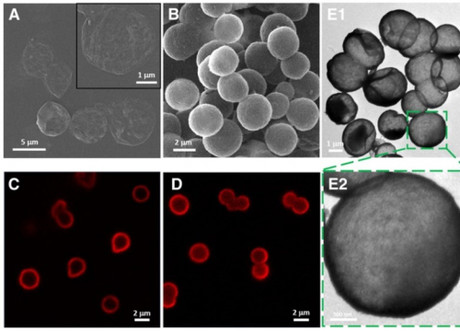
To solve this problem, the TPU team decided to utilise polymeric and hybrid silica-coated capsules (SiO2), which they had been developing in collaboration with the Pavlov First Saint Petersburg State Medical University of St. Petersburg. Each capsule measures 2–2.5 μm.
The process begins with each capsule being loaded with genetic material (biologically active substances), ie, miRNA, mRNA and plasmid DNA. They are then delivered to the target cell, which absorbs them through micropinocytosis: a process in which cells capture relatively big particles.
Now based in the cytoplasm of cells, the microcapsules are finally able to release their content. The shell itself gradually dissolves.
“Delivery efficiency with these microcapsules is achieved due to low toxicity,” said Timin. “The study showed that over 90% of cells survive after transfection. Thus, the percentage of edited cells became significantly higher in comparison when liposomes were used for transfection.”
The results obtained allow the scientists to conclude that their microcapsules represent promising vectors for application of genome-editing tools. The next stage, according to the researchers, will be the application of CRISPR-Cas9 using microcapsules to deliver genetic material in in vivo studies.
A pre-emptive approach to treating leukaemia relapse
The monitoring of measurable residual disease (MRD), medication and low-dose chemotherapy is...
Long COVID abnormalities appear to resolve over time
Researchers at UNSW's Kirby Institute have shown that biomarkers in long COVID patients have...
RNA-targeted therapy shows promise for childhood dementia
Scientists have shown that a new RNA-targeted therapy can halt the progression of a specific type...







