Are your results reproducible?

The scientific community is currently facing a reproducibility crisis. Over 70% of researchers are failing to reproduce published experiments1.
With so much research built on previous work, this represents a substantial amount of time and funding wasted because of reproducibility issues. Some estimates put the cost of unreliable, irreproducible research as high as $28 million each year in the US2. Although there are several causes of this “crisis”, it can’t be denied that the quality of scientific reagents is a core issue we need to address.
Reagents are the essential building blocks for all life science research. It’s crucial that reagents work as specified and provide accuracy and consistency so that scientists can generate robust data. One push in the right direction is the use of recombinant technology in the production of antibodies.
Abcam’s catalogue has seen a massive increase in the number of recombinant antibodies on offer. These are developed in vitro using a synthetically produced gene construct, so antibody production is controlled, reliable, and avoids common issues such as gene mutations. We currently offer a range of over 13,000 recombinant monoclonal antibodies, all ensuring high specificity and batch-to-batch reproducibility.
Scientists need products that they can rely on; products that work as expected and don’t lead to wasted time and funding. Vendors need to rigorously test reagents for reproducibility and specificity across a range of applications. We’ve heavily invested in knockout (KO) validation techniques — the gold standard in antibody validation — to give our customers confidence in the products they buy from us.
KO-validation confirms antibody specificity by testing the antibody of interest on a KO sample or cell line that doesn’t express the target protein. KO-validation serves as a true negative control to help confirm specificity to the protein of interest. We’re conducting this KO-validation program at a scale beyond anyone else in the industry with over 1,600 KO validated antibodies in our catalog and growing.
To ensure that researchers using kits rather than antibodies alone benefit from our specificity testing, we use the same validated antibodies in our range of immunoassay kits as well. Our SimpleStep ELISA® and multiplex FirePlex® kits, for example, make extensive use of our own recombinant monoclonal antibody pairs, to deliver specific and reproducible data within and between batches.
Transparent product data gives scientists confidence that the reagents they purchase have worked well in the hands of their peers. We upload all customer reviews to the Abcam site — there are no edits, whether a review is positive or negative. We continually test existing products in our catalogue, adding new testing data and removing outdated products, so researchers always have the best available options and information. On our website, you can also find information on how our antibodies compare to equivalent alternatives from our top competitors (see figure 1 for an example). We carry out these tests in an entirely fair and comparable manner showing all the data we get, even if the competitor reagents are just as good as our own.
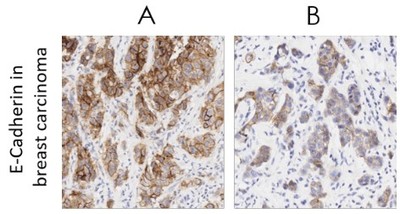
We’re trying to change the problem of reproducibility and advance scientific research by setting new standards for reagent validation and testing. Our products perform to exceptionally high standards, and that’s why researchers are choosing to buy their reagents from us. Since January 2015 Abcam products were cited in 33% of all life science publications3. We’re driven to improve antibody reliability and specificity, and with the help of the scientific community and other vendors, the reproducibility crisis could soon become a thing of the past.
For more information: www.abcam.com/qualityvalidation.
References
- Baker, M. Nature 533, 452–454 (2016)
- Freedman L.P. et al. PLOS Biology (2018)
- CiteAb: https://www.citeab.com/ accessed 14/09/2018
Safer Labs for SPREE
To maintain its benchmark in photovoltaic and renewable energy research, UNSW's School of...
Certified Calibration Standards vs Primary Gravimetric Standards
BOC's world-class laboratory allows for a database of over 5,000 individual mixtures, all...
Accurate solution phase affinity profiling of a SARS-CoV-2 antibody in serum
The ability to accurately characterize the immune response against SARS-CoV-2 is of vital...




