Antibody's unexpected response to Strep A infection
Scientists at Lund University have discovered a rare form of antibody binding that leads to an effective immune response against group A streptococcus bacteria. Their research, published in the journal EMBO Molecular Medicine, could explain why so many group A strep vaccines have failed.
Group A streptococci have several ways in which they evade the body’s immune system and can cause strep throat, scarlet fever, sepsis, swine pox and skin infections. Antibiotics currently work against these bacteria, but should they become resistant, they will pose a major public health threat.
One strategy that the scientific community uses to find new ways of fighting bacterial infections is to create target-seeking antibodies, whereby the antibodies that the body’s immune system produces in the event of an infection are mapped and their effect on the immune system is studied. In this way, antibodies can be identified that can be used both for preventive treatment and for treatment during an ongoing infection.
However, this is a challenging process, and many attempts to develop antibody-based treatments against Strep A have failed. The new study shows an unexpected way that antibodies interact with group A streptococci and, more specifically, how they hook onto the probably most important bacterial protein — the M protein — on the cell surface.
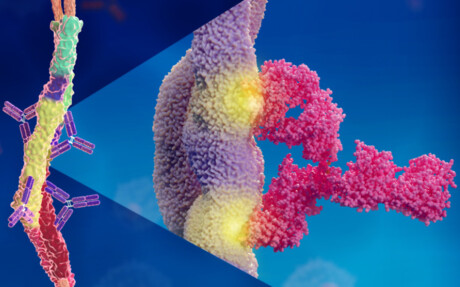
“We found that it happens in a way that has never been described before,” said study co-author Pontus Nordenfelt. “Normally, an antibody binds via one of its two Y arms to its target protein at a single site, regardless of which of the two arms is used for binding. But what we have seen … is that the two Y arms can recognise and hook on to two different places on the same target protein.”
This means that the two arms of the antibody — which are identical — can bind to two different sites on a target protein. It turns out that it is precisely this type of binding that is required for effective protection, and could explain why so many vaccine attempts have been unsuccessful. It could also be a reason why the bacteria manage to escape the immune system.
It has long been known that the streptococcal bacteria’s M protein is of great importance for how disease develops in the body. Finding an antibody that attaches to this protein, thereby flagging it up to the immune system, can prevent the bacteria from infecting the body’s cells. Since we know that the human body can fight the infection, such antibodies exist, but it is hard to locate them.
The researchers focused on examining antibodies in former Strep A patients, and managed to identify three monoclonal antibodies in a patient who recovered from infection. Monoclonal antibodies are identical copies of each other, and in this case target a single protein (the M protein) of the group A streptococci. The researchers then investigated in animal studies whether it is possible to use the antibodies to strengthen the immune system in its fight against group A streptococcus. It turned out that the antibody with the newly discovered binding mechanism produced a strong immune response against the bacteria.
“This opens up possibilities where previous vaccine attempts have failed and means that the monoclonal antibody we used has the potential to protect against infection,” said study co-author Wael Bahnan.
Fluorescent spray detects fingerprints at crime scenes
Scientists have developed a water-soluble, non-toxic fluorescent spray that makes fingerprints...
NIST develops urine standard for kidney disease diagnosis
In order for doctors to diagnose kidney disease and other conditions that affect kidney function,...
Liquid reagent inactivates SARS-CoV-2 in patient samples
The unique composition of the reagent could extract RNA in patient samples in little as...







