Three-person IVF: mitochondrial donation approved in UK
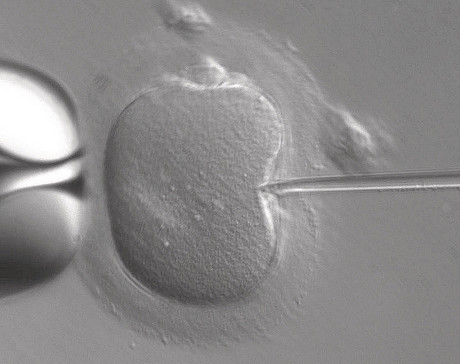
The United Kingdom has become the first country to officially authorise the clinical use of mitochondrial donation IVF to enable healthy babies to be born to women carrying deadly mitochondrial disease (mito), which starves major organs of energy. The ability to walk, run or even just stand up unaided can be a daily struggle for people with mito, which has few treatments and no cure.
The Human Fertilisation and Embryology Authority (HFEA) met in London yesterday to consider recommendations from an expert panel to license so-called ‘three-person baby IVF’, in which a future baby’s mitochondrial DNA comes from a third party. The panel recommended that the technique could be used cautiously for risk reduction treatments in certain cases where alternative treatments would be of little or no benefit to mothers at risk of passing mitochondrial disease onto their children.
“The report to the HFEA concludes that recent scientific advances have sufficiently addressed the potential carryover of faulty mitochondrial DNA,” said Professor David Thorburn, head of mitochondrial research at Murdoch Childrens Research Institute, who made a submission to the independent panel. “It also recommends numerous safeguards such as carefully selecting women to undergo the procedure as a clinical risk reduction treatment, providing full information about potential limitations and risk, and undertaking genetic testing when the embryo is at 15 weeks’ gestation.
“It is important to note that the donor mitochondrial DNA only replaces 37 mtDNA genes — contributing about 0.1% of the baby’s genetic make-up — compared with approximately 20,000 genes in the nucleus, which are not replaced.”
Parliament passed regulations permitting maternal spindle transfer (MST) and pronuclear transfer (PNT) in February 2015, and the regulatory framework has been in place since October 2015, but clinics had been advised to wait until after the HFEA had considered the panel’s recommendations before applying for permission to offer mitochondrial donation to patients. The HFEA has now approved such donation in certain, specific cases, meaning that specialist IVF clinics wanting to MST or PNT to patients may apply to the authority for permission to do so.
“After a lot of hard work and invaluable advice from the expert panel, who reviewed the development, safety and efficacy of these techniques over five years and four reports, we feel now is the right time to carefully introduce this new treatment in the limited circumstances recommended by the panel,” said HFEA Chair Sally Cheshire.
With the treatment now approved in the UK, the Australian Mitochondrial Disease Foundation (AMDF) is calling on the Australian Government to follow suit.
“At least 60 Australian babies born each year suffer with severe and life-threatening forms of mitochondrial disease that could be prevented by using the mother’s and father’s nuclear DNA and replacing the mother’s defective mitochondrial DNA with healthy mitochondrial DNA from a donor egg,” said AMDF CEO Sean Murray.
“Based on the extensive evidence available, the Australian Mitochondrial Disease Foundation believes the potential benefits of mitochondrial replacement outweigh the risks for unborn children who would otherwise almost certainly develop potentially fatal mitochondrial disease.”
Melatonin helps to prevent obesity, studies suggest
In an experiment carried out in rats, chronic administration of melatonin prevented obesity to a...
Personality influences the expression of our genes
An international research team has used artificial intelligence to show that our personalities...
Pig hearts kept alive outside the body for 24 hours
A major hurdle for human heart transplantation is the limited storage time of the donor heart...







