Bespoke analytical technology platforms improve testing process
A successful collaboration between automation systems developer Astech Projects and a research-based pharmaceutical and healthcare company has been detailed in the poster ‘Technology Implementation for Analysis of Inhaled Products’.
Having been recently presented at the Drug Delivery to the Lungs (DDL) conference and exhibition, the poster details how bespoke analytical technology platforms in support of dry powder inhaler (DPI) testing have been implemented within the GlaxoSmithKline (GSK) inhaled testing laboratories using a quality by design approach. The process has been summarised below.
Novel Emitted Dose (nED)
The dry powder inhaler (DPI) nED is a manual apparatus that recovers the emitted dose from DPI devices. During the emitted dose test, a DPI device is primed and clamped into a firing station sealed to a DPI Dose Collector and an airflow drawn through the device via a critical flow controller. The collected dose is recovered via a washing station by pumping solvent through the dose collector into a volumetric flask for analysis. Drying and re-preparation is performed using a Dose Collector Drying Station. This technology greatly improved robustness of the testing and reliability of data as well as delivering a much more efficient and cost-effective procedure over the manual procedure.
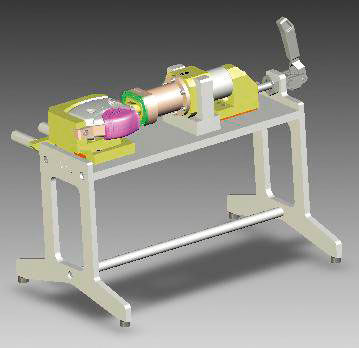
Blister Sampling Apparatus (BSA)
The BSA facilitates the recovery of powder blend from single blisters from a DPI strip for either the content uniformity or bulk assay procedures. The DPI strip is clamped onto the BSA, the blister is punched out of the strip and pierced before metered solvent is dispensed from a solvent reservo through the blister and delivered into a recovery flask for processing and analysis. This system meant that significantly more data points could be generated in a safer, cost-effective and more reliable way.
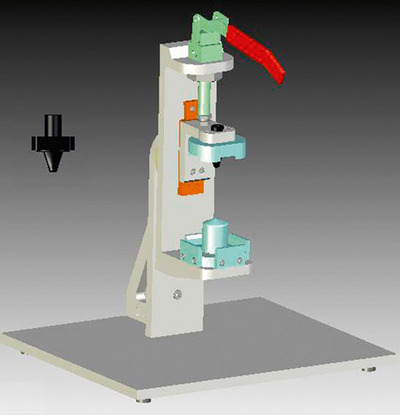
Next Generation Dose Sampling Introduction (NDSI)
The NDSI system automates either waste firing or collection of the powder within the Next Generation Impactor (NGI). The device holder is identical to that on the AED (see below) and nED and thus minimises any potential variability in the sealing of the device to the NGI USP Throat. This system requires minimal human intervention and complements the NSRS, improving robustness of the NGI procedure.

Next Generation Impactor Sample Recovery System (NSRS)
The NSRS is designed to quantitatively recover drug particles from the NGI with solvent and deliver the sample solutions into HPLC vials for analysis. The hardware has nine individual plumbing subsystems, each an isolated channel that recovers, collects and cleans a set of NGI collection cups and related components. The throat, mouthpiece, collection cups 1 to 7 and Micro Orifice Collector (MOC) are each rinsed with a single aliquot of solvent. Recovery method parameters are specified in the user-defined methods. All operations and events of the NSRS are recorded in the software database and can be printed in preformatted reports. Particle size distributions comprising all stages of the Impactor can be consistently generated both during product development and routine quality control.

Automated Emitted Dose (AED)
The AED fully automates the DPI product content uniformity test. Once the user loads the devices, appropriate solvents and initiates the correct program, the system will waste fire and collect the defined blisters. The collectors are rinsed with solvent and HPLC autosampler vials containing sample solution are prepared. With less human intervention, the risk of analytical error is significantly reduced.

Results
In comparison to the manual procedures previously employed, the platforms increased throughput by substantially reducing the time taken to perform 100 samples and minimising analytical variability. In addition, use of the platforms decreased the volume of solvent needed for testing, reducing the waste streams and resulting in greener, more cost-effective testing.
Conclusion
Technology platforms have been implemented throughout the product development lifecycle, improving throughput, efficiency, safety and minimising analytical variability. The platforms form a strategy to support and justify product specifications and improve the science and understanding of inhaled products. They will be implemented across the GSK network as part of method/technology transfer prior to product, ensuring that the analysis of Inhaled products across the network is rugged and representative of the true quality of the product.
By Geoff Daniels and Andrew Rice, GlaxoSmithKline
From assistance to agency in biopharma R&D: benefits of next-gen AI-native lab notebooks
The next generation of AI-native lab notebooks has the potential to turn AI into an active...
Images reveal "incredible" detail of industrial micro-CT scanner
Images of intricate biological structures and everyday items have revealed the capabilities of a...
MRI scanner to advance medical breakthroughs at Monash
Siemens Healthineers' MAGNETOM Cima.X 3T is claimed to be Victoria's most advanced,...




