Ocean acidification may be damaging sharks' teeth

Sharks can famously replace their teeth, with new ones always growing as they’re using up the current set. But the ability to regrow teeth might not be enough to ensure they can withstand the pressures of a warming world where oceans are getting more acidic, with a new study published in the journal Frontiers in Marine Science revealing that more acidic oceans lead to more brittle and weaker teeth.
“Shark teeth, despite being composed of highly mineralised phosphates, are still vulnerable to corrosion under future ocean acidification scenarios,” said Maximilian Baum, a biologist at Heinrich Heine University Düsseldorf (HHU) and first author on the study. “They are high developed weapons built for cutting flesh, not resisting ocean acid. Our results show just how vulnerable even nature’s sharpest weapons can be.”
Ocean acidification is a process during which the ocean’s pH value keeps decreasing, resulting in more acidic water, and is mostly driven by the release of human-generated carbon dioxide (CO2). Currently, the average pH of the world’s oceans is 8.1 — but by 2300, it is expected to be 7.3, making it almost 10 times more acidic than it currently is.
For their study, the researchers used these two pH values to examine the effects of more and less acidic water on the teeth of blacktip reef sharks. Divers collected more than 600 discarded teeth from an aquarium housing the sharks. 16 teeth — those that were completely intact and undamaged — were used for the pH experiment, while 36 more teeth were used to measure before and after circumference. The teeth were incubated for eight weeks in separate 20 L tanks, with the teeth exposed to more acidic water found to be significantly more damaged.
“We observed visible surface damage, such as cracks and holes, increased root corrosion, and structural degradation,” said senior author Professor Sebastian Fraune, who heads the Zoology and Organismic Interactions institute at HHU. Tooth circumference was also greater at lower pH levels, but this does not mean the teeth actually grew; rather, the surface structure became more irregular, resulting in it appearing larger on 2D images. While an altered tooth surface may improve cutting efficiency, it potentially also makes teeth structurally weaker and more prone to break.
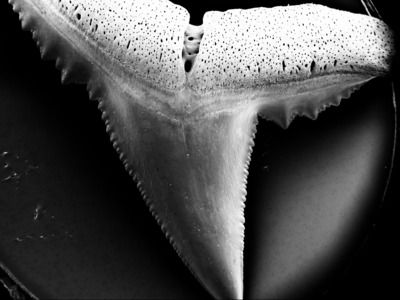
Blacktip reef sharks must swim with their mouths permanently open to be able to breathe, so their teeth are constantly exposed to water. If the water is too acidic, the teeth automatically take damage, especially if acidification intensifies, the researchers said.
“Even moderate drops in pH could affect more sensitive species with slow tooth replication circles or have cumulative impacts over time,” Baum said. “Maintaining ocean pH near the current average of 8.1 could be critical for the physical integrity of predators’ tools.”
That said, the study only looked at discarded teeth of non-living mineralised tissue, which means repair processes that may happen in living organisms could not be considered. Fraune explained, “In living sharks, the situation may be more complex. They could potentially remineralise or replace damaged teeth faster, but the energy costs of this would be probably higher in acidified waters.”
The researchers thus acknowledge that future studies should examine changes to teeth, their chemical structure, and mechanical resilience in live sharks. Nevertheless, the current study shows that microscopic damage might be enough to pose a serious problem for animals depending on their teeth for survival.
“It’s a reminder that climate change impacts cascade through entire food webs and ecosystems,” Baum concluded.
Liquid handling with machine learning — the perfect screening combo?
In December, scientists at St. Jude Children's Research Hospital made public a screening...
Best practices for safe centrifugation in the laboratory
The majority of all centrifuge accidents are understood to result from user error. These tips...
Nature-inspired filtration system recovers critical resources
A filtration system that can recover untapped critical resources such as copper and lithium from...



