How early embryos change shape
Researchers at EMBL Australia, based at the Australian Regenerative Medicine Institute at Monash University, have found a new mechanism controlling the process of how embryos change shape.
Embryos comprising eight cells change their shape in a process that is vital to their survival. The cells became elongated and compacted against each other, before returning to their rounded shape and dividing again and again. When compaction does not occur the embryos tend not to survive, and the timing of compaction has been linked to success in IVF treatments.
In a paper published in the journal Nature Cell Biology, the researchers noted that “it remains unclear how round cells elongate to form a compacted embryo”. So they used live imaging technology and microinjected fluorescent markers to capture this process in mouse embryos.
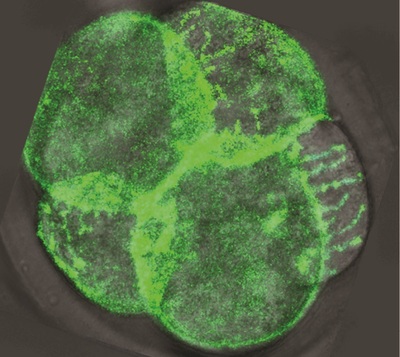
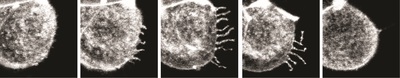
Dr Juan Carlos Fierro-González, who is co-lead author of the paper with Dr Melanie White, said the images reveal “arm-like structures called filopodia appearing on the outer membrane of some cells during the 8-cell stage, and it is these filopodia that are responsible for contorting cell shape and forming the embryo’s first tissue-like layers”.
“For the first time, we have been able to watch as filopodia reach out and grab neighbouring cells, pulling them closer and elongating the cell membranes. We think that this enables the cells to effectively compact, as their new non-rounded shape makes the most of the available space.”
Next, said Dr Fierro-González, the researchers “saw the filopodia retract as they released their grip on neighbouring cells, allowing them to return to a somewhat rounded shape before they continued on their journey of cell division”. The team observed that cell division never occurred while filopodia were extended over the cells, but only once the filopodia had retracted.
These observations have led the researchers to believe that the filopodia provide the necessary surface tension to allow the cells to undergo expansion and compaction. As team leader Dr Nicolas Plachta describes it, “These arm-like filopodia are hugging the cells, squeezing them into shape.”
Dr Plachta’s team are working in partnership with the Monash School of Engineering to improve implantation success rates for human embryos - an area into which this new knowledge can be applied.
“Now that we know what controls early development, we are designing non-invasive imaging approaches to see if human embryos used in IVF form normal filopodia and undergo normal compaction,” said Dr Plachta. “This could help us choose which embryos should or shouldn’t be implanted back in the uterus.”
Blood test could be used to diagnose Parkinson's earlier
Researchers have developed a new method that requires only a blood draw, offering a non-invasive...
Cord blood test could predict a baby's risk of type 2 diabetes
By analysing the DNA in cord blood from babies born to mothers with gestational diabetes,...
DNA analysis device built with a basic 3D printer
The Do-It-Yourself Nucleic Acid Fluorometer, or DIYNAFLUOR, is a portable device that measures...



