Quantum simulation of chemical dynamics achieved
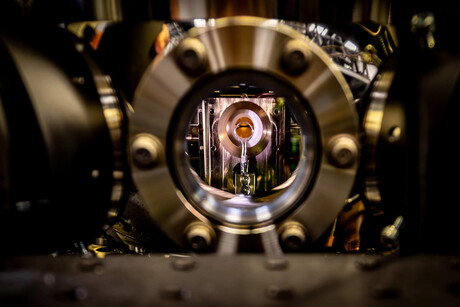
Understanding in real time how atoms interact to form new compounds or interact with light has long been expected as a potential application of quantum technology. Now researchers at The University of Sydney say they have successfully performed a quantum simulation of chemical dynamics with real molecules for the first time, with their results published in the Journal of the American Chemical Society.
Until now, quantum computers have been limited to calculating static properties of molecules — such as their energies — leaving the dynamic, time-evolving processes largely inaccessible given their complexity. However, the new research pushes the frontier by simulating how molecules behave when excited by light — a process involving ultrafast electronic and vibrational changes that classical computers struggle to model accurately or efficiently. Quantum chemist Professor Ivan Kassal, from The University of Sydney Nano Institute and School of Chemistry, compares this to hiking up a mountain.
“It is one thing to understand your starting point, your end point, and how high you’ll need to climb. But this doesn’t help you understand the path you will take,” he said.
“Our new approach allows us to simulate the full dynamics of an interaction between light and chemical bonds. It’s like understanding the position and energy of the mountain hiker at any time point of their journey through the mountains.”
The new work leverages a novel, highly resource-efficient encoding scheme implemented on a trapped-ion quantum computer in the Sydney Nanoscience Hub. It also builds on an earlier study from 2023, where the research team simulated abstract generic quantum dynamics by slowing the process down a factor of 100 billion times.
“We have taken that study and applied its approach to the dynamics of three different molecules after they’ve absorbed light,” said Physics Horizon Fellow Dr Tingrei Tan.
“It is possible to simulate the interactions for these particular molecules using classical supercomputers. But more complex molecules will be beyond their capabilities. Quantum tech will be able to simulate such complexity that is beyond all classical capability.”
Imagine witnessing a molecule absorb a photon, vibrate and undergo rapid electronic transition — all encoded in a quantum simulation that unfolds over a staggering time-dilation factor of 100 billion (1011). This means the quantum simulation runs on an accessible timescale of milliseconds, while faithfully reproducing the ultrafast chemical events occurring in femtoseconds (10–15).
Significantly, unlike the earlier work of this team and other research efforts that only modelled abstract dynamical systems, this study simulates real molecules — demonstrating the method’s capacity to mimic actual chemical processes. In this instance, the simulation was of light interacting with allene (C3H4), butatriene (C4H4) and pyrazine (C4N2H4).
What makes this achievement particularly groundbreaking is the efficiency of the approach. The team employed an analog quantum simulation method using just a single trapped ion — a fraction of the hardware resources needed by traditional digital quantum computers.
“Performing the same simulation using a more conventional approach in quantum computing would require 11 perfect qubits and 300,000 flawless entangling gates,” Kassal said. “Our approach is about a million times more resource-efficient, enabling complex chemical dynamics to be studied with far fewer resources than previously thought possible.”
This development holds great promise for simulating chemical reactions and chemical dynamics in any situation where light is involved: from photosynthesis and DNA damage caused by UV radiation to advanced medical therapies like photodynamic therapy for cancers or skin disorders and sunscreens.
“In all these cases, the ultrafast photo-induced dynamics are poorly understood,” Tan said. “Having accurate simulation tools will accelerate the discovery of new materials, drugs or other photoactive molecules.”
This experimental breakthrough thus demonstrates that quantum simulation of real, complex molecules is within practical reach — an achievement that could dramatically accelerate scientific discovery in chemistry and beyond. By harnessing the power of quantum mechanics in a highly resource-efficient way, the University of Sydney team has opened a promising new pathway towards understanding the ultrafast chemical processes that underpin vital biological functions, energy solutions and materials innovation.
From assistance to agency in biopharma R&D: benefits of next-gen AI-native lab notebooks
The next generation of AI-native lab notebooks has the potential to turn AI into an active...
Images reveal "incredible" detail of industrial micro-CT scanner
Images of intricate biological structures and everyday items have revealed the capabilities of a...
MRI scanner to advance medical breakthroughs at Monash
Siemens Healthineers' MAGNETOM Cima.X 3T is claimed to be Victoria's most advanced,...



