Archer completes potassium sensing alpha prototype
Quantum technology company Archer Materials Limited has developed an early Biochip prototype designed to detect chronic kidney disease.
The company announced the completion of its alpha prototype and laboratory demonstration of its blood potassium sensor with integrated Biochip in January; a milestone, the company said in a statement, that “represents the first major step in translating Archer’s Biochip technology from individual laboratory devices into a product suitable for clinical workflows and, ultimately, point of care and at-home potassium monitoring”.
“For the first time, we have integrated our potassium sensing Biochip with fluidics and electronics into a single packaged system, while maintaining clinical-grade accuracy using patient-derived samples. This represents a major technical milestone for Archer’s Biochip program. The achievement significantly derisks the technology paving the way for developing a full (beta) prototype in 2026,” Archer CEO Dr Simon Ruffell said.
“Archer’s testing of the early prototype shows that the Biochip technology meets clinical accuracy requirements when integrated into a prototype cartridge capable of handling a drop of blood and the product-ready electronic readout system.”
Potassium measurement accuracy within ±0.3 mM in blood samples was demonstrated by the Biochip prototype, Archer said, which is in alignment with Clinical Laboratory Improvement Amendments (CLIA) requirements for equivalent testing in a pathology lab and matches the previous laboratory experimental system results.
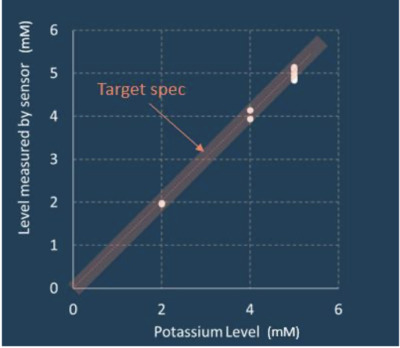
Incorporating the Biochip, fluidics and electronics into a single test system, Archer said its first integrated alpha prototype delivers the following outcomes:
- clinical-grade potassium measurement accuracy maintained while operating in a fully prototype environment;
- qualified micro-fluidics capable of performing accurate potassium testing using only 10μL of blood, a volume consistent with standard finger-prick tests and eliminating the need for finger ‘milking’, which is a key requirement for patient usability and eventual at-home adoption; and
- verified prototype electronics, confirming system-level functionality and stable performance across repeated measurements — ready for integration into final product.
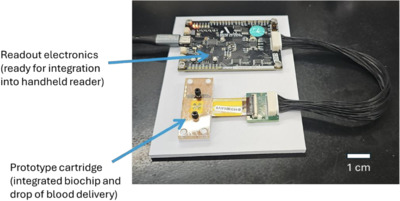
The beta prototype will form the basis of negotiations with large MedTech companies regarding potential licensing deals as well as engagement with contract medical device manufacturers, the company said.
The University of Sydney formalises cervical cancer elimination partnership
The success of a cervical cancer elimination program has led to the signing of a memorandum of...
Noxopharm says paper reveals science behind its immune system platform
Clinical-stage Australian biotech company Noxopharm Limited says a Nature Immunology...
Neurosensing/neurostimulation implants session to be held on Monday
On Monday, a session at UNSW Sydney will include people who are benefiting from bioelectronics...



