A rift in the retina helps transplanted cells better integrate
Johns Hopkins Medicine researchers have found that removing a membrane that lines the back of the eye may improve the success rate for regrowing nerve cells damaged by blinding diseases. Their findings, published in the journal Stem Cell Reports, are aimed at discovering new ways to reverse vision loss caused by glaucoma and other diseases that affect the optic nerve, the information highway from the eye to the brain.
“The idea of restoring vision to someone who has lost it from optic nerve disease has been considered science fiction for decades — but in the last five years, stem cell biology has reached a point where it’s feasible,” said Thomas Johnson, an assistant professor of ophthalmology at the Johns Hopkins University School of Medicine.
A human eye has more than 1 million small nerve cells, called retinal ganglion cells, that transmit signals from light-collecting cells called photoreceptors in the back of the eye to the brain. Retinal ganglion cells send out long arms, or axons, that bundle together with other retinal ganglion cell projections, forming the optic nerve that leads to the brain.
When the eye is subjected to high pressure, as occurs in glaucoma, it damages and eventually kills retinal ganglion cells. In other conditions, inflammation, blocked blood vessels or tumours can kill retinal ganglion cells. Once they die, retinal ganglion cells don’t regenerate.
“That’s why it is so important to detect glaucoma early,” Asst Prof Johnson said. “We know a lot about how to treat glaucoma and help nerve cells survive an injury, but once the cells die off, the damage to someone’s vision becomes permanent.”
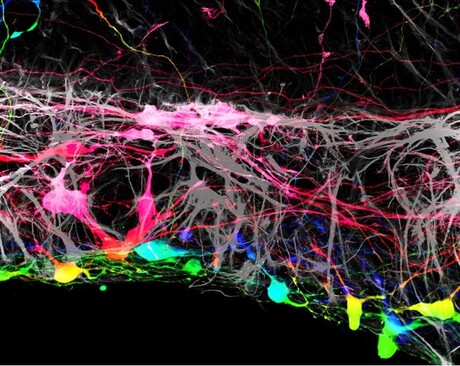
Asst Prof Johnson is a member of a team of researchers at the Johns Hopkins Wilmer Eye Institute looking for ways to repair or replace lost optic neurons by growing new cells. In their current study, he and his team grew mouse retinas in a laboratory dish and tracked what happens when they added human retinal ganglion cells, derived from human embryonic stem cells, to the surface of the mouse retinas. They found that most of the transplanted human cells were unable to integrate into the retinal tissue, which contains several layers of cells.
“The transplanted cells clumped together rather than dispersing from one another like on a living retina,” Asst Prof Johnson said.
However, the researchers found that a small number of transplanted retinal cells were able to settle uniformly into certain areas of the mouse retina. Looking more closely, the areas where the transplanted cells integrated well aligned with locations where the researchers had to make incisions into the mouse retinas to get them to lie flat in the culture dish. At these incision points, some of the transplanted cells were able to crawl into the retina and integrate themselves in the proper place within the tissue.
“This suggested that there was some type of barrier that had been broken by these incisions,” Asst Prof Johnson said. “If we could find a way to remove it, we may have more success with transplantation.”
It turns out that the barrier is a well-known anatomical structure of the retina, called the internal limiting membrane. It’s a translucent connective tissue created by the retina’s cells to separate the fluid of the eye from the retina.
After using an enzyme to loosen the connective fibres of the internal limiting membrane, the researchers removed the membrane and applied the transplanted human cells to the retinas. They found that most of the transplanted retinal ganglion cells grew in a more normal pattern, integrating themselves more fully. The transplanted cells also showed signs of establishing new nerve connections to the rest of the retinal structure when compared with retinas that had intact membranes.
“These findings suggest that altering the internal limiting membrane may be a necessary step in our aim to regrow new cells in damaged retinas,” Asst Prof Johnson said. He and his team plan to continue investigating the development of transplanted retinal ganglion cells to determine the factors they need to function once integrated into the retina.
Please follow us and share on Twitter and Facebook. You can also subscribe for FREE to our weekly newsletters and bimonthly magazine.
Aussie biotech to manufacture mRNA paediatric brain cancer vaccines
A Queensland-based biotechnology company will manufacture personalised mRNA paediatric brain...
Who's afraid of killer whales? — white sharks and prolonged absences
Is killer whale predation the sole driver of white shark long absence? Australian researchers...
Five scenarios for the future of Antarctic life
A team of Australian and international researchers have predicted five possible outcomes for how...




