Ebola vaccine proves highly effective
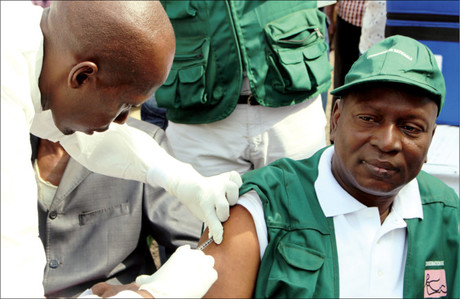
An experimental Ebola vaccine has proven highly protective against the deadly virus in a major trial conducted in Guinea. The trial was led by the World Health Organization (WHO), Guinea’s Ministry of Health, Médecins Sans Frontières, the Norwegian Institute of Public Health and other international partners.
The vaccine, called rVSV-ZEBOV, was studied in a trial involving 11,841 people in the coastal region of Basse-Guinée, the area of Guinea still experiencing new Ebola cases back when the trial was conducted in 2015. It utilised a so-called ‘ring vaccination’ approach — the same method used to eradicate smallpox — in which the research team traced all people who may have been in contact with new Ebola cases within the previous three weeks, as well as certain ‘contacts of contacts’. A total of 117 clusters (or ‘rings’) were identified, each made up of an average of 80 people.
Initially, rings were randomised to receive the vaccine either immediately or after a three-week delay, and only adults over 18 years were offered the vaccine. After interim results were published showing the vaccine’s efficacy, all rings were offered the vaccine immediately and the trial was also opened to children older than six years. Among the 5837 people who received the vaccine, no Ebola cases were recorded 10 days or more after vaccination; in comparison, there were 23 cases among those who did not receive the vaccine.
Published in The Lancet, the study additionally showed that unvaccinated people in the rings were indirectly protected from Ebola virus through the ring vaccination approach (so-called ‘herd immunity’). However, the authors note that the trial was not designed to measure this effect, so more research will be needed. Additional studies will also provide more data on the safety of the vaccine in children and other vulnerable populations such as people with HIV.
The vaccine’s manufacturer, Merck Sharp & Dohme, this year received Breakthrough Therapy Designation from the US Food and Drug Administration and PRIME status from the European Medicines Agency, enabling faster regulatory review of the vaccine once it is submitted. Merck has committed to submit the vaccine for licensure by the end of 2017, while GAVI, the Vaccine Alliance has provided US$5 million to Merck towards the future procurement of the vaccine once it is approved, prequalified and recommended by WHO.
How a tiny worm changed a decade of scientific thinking
A tiny roundworm has helped University of Queensland scientists rethink the way sensory nerve...
A scientist, a cooler and a long-haul flight in the fight against MND
Early in January 2026, neuroscientist Dr Rachael Dunlop boarded a flight from Sydney to the US...
Mini lung organoids could help test new treatments
Scientists have developed a simple method for automated the manufacturing of lung organoids...



