Eight babies born in UK following mitochondrial donation

Eight children in the UK have been born following a pioneering licensed IVF technique to reduce the risk of mitochondrial diseases, known as mitochondrial donation. The UK was the first country to approve laws to allow the use of this groundbreaking technology in 2015, followed by Australia in 2022.
Mitochondria produce the energy required for life and contain a small piece of DNA that only encodes some of the instructions required for energy production. Harmful mutations in mitochondrial DNA can result in reduced availability of energy, particularly affecting tissues that have high energy demands (such as the heart, muscle and brain). Mitochondrial DNA is maternally inherited, meaning mitochondrial diseases are passed from mother to child (males can be affected but do not pass on the disease), and despite years of research there is still no cure. Attention has therefore turned to IVF-based technologies to reduce the risk of disease by limiting transmission of disease-causing mitochondrial DNA mutations from mother to child — with so-called ‘pronuclear transfer’ (PNT) showing particular promise.
PNT is performed after the egg is fertilised. It involves transplanting the nuclear genome (which contains all the genes essential for our individual characteristics, such as hair colour and height) from an egg carrying a mitochondrial DNA mutation to an egg donated by an unaffected woman that has had its nuclear genome removed. The resulting embryo inherits its parents’ nuclear DNA, but the mitochondrial DNA is inherited predominantly from the donated egg.
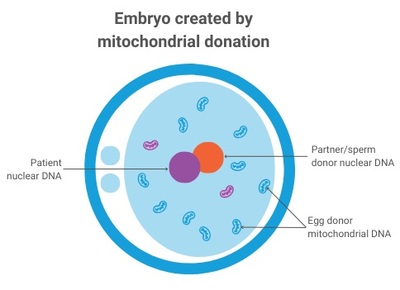
PNT was pioneered in human eggs over almost two decades by a team based at Newcastle University and the Newcastle upon Tyne Hospitals NHS Foundation Trust, led by Professor Mary Herbert. It is now being offered to prospective parents through a research study, as part of an integrated program that also includes pre-implantation genetic testing (PGT) — a procedure that helps couples avoid passing on genetic conditions by testing their embryos for such conditions. In accordance with regulations, PNT is offered only to those women who are unlikely to benefit from PGT treatment.
The team’s work to date has been published across two papers in The New England Journal of Medicine (NEJM).
The reproductive outcomes paper
At the time of reporting, clinical pregnancies were confirmed in eight of 22 (36%) patients who underwent PNT as part of the study and 16 of 39 (41%) of patients who underwent PGT. PNT has so far resulted in eight births to seven women (including one set of identical twins) and one further pregnancy, while PGT has resulted in 18 births.
Despite being born to mothers at high risk of transmitting serious disease caused by mutations in mitochondrial DNA, levels of disease-causing mitochondrial DNA mutations in the PNT newborns’ blood were either undetectable or present at low levels. According to the researchers, the presence of such mutations results from carryover of maternal mitochondria surrounding the nuclear DNA at the time of transplantation; a known limitation of mitochondrial donation technologies.
“The findings give grounds for optimism,” said Herbert, who was lead author of the reproductive outcomes paper. “However, research to better understand the limitations of mitochondrial donation technologies will be essential to further improve treatment outcomes.
“Mitochondrial donation technologies are currently regarded as risk-reduction treatments owing to carryover of maternal mitochondrial DNA during the mitochondrial donation procedure. Our ongoing research seeks to bridge the gap between risk reduction and prevention of mitochondrial DNA disease by addressing this problem.”
The clinical outcomes paper
All eight PNT babies were healthy at birth (whether born by normal vaginal delivery or elective caesarean section), had normal weight for their gestational age, and were described as developing normally and meeting relevant milestones. The team noted that three babies overcame separate early health issues that they were not able to attribute directly to mitochondrial donation; these included some brief startles, which resolved without treatment; high blood fats (which also affected the mother during pregnancy) and cardiac arrhythmia (treated with a low-fat diet and anti-arrhythmic medication, respectively); and a urinary tract infection that responded quickly to antibiotic treatment.
The level of disease-causing mitochondrial DNA mutation was measured in blood and urine cells and was undetectable in five babies. Three babies had low levels of disease-causing mitochondrial DNA mutations — 5 and 9%, 12 and 13%, and 16 and 20% in blood and urine respectively. These levels are well below the 80% level required for clinical disease for these mutations, suggesting they would not be responsible for any of the children’s health conditions. At follow-up at 18 months, the level of the disease-causing mutation in the child with 5 and 9% was undetectable in blood and urine. The team will continue to offer assessments up to the age of five years, with the aim of detecting any patterns in childhood conditions.
“While longer term follow-up of children born following mitochondrial donation is of paramount importance, these early results are very encouraging,” said Bobby McFarland, Director of the NHS Highly Specialised Service for Rare Mitochondrial Disorders (Newcastle Hospitals NHS Foundation Trust) and Professor of Paediatric Mitochondrial Medicine at Newcastle University, who serves as first author on the clinical outcomes paper.
“We believe the follow-up process we have put in place is thorough, since it allows us to detect and review even minor health conditions in children born after pronuclear transfer, such as a urinary tract infection.”
What does this mean for Australia?
Herbert relocated to Melbourne’s Monash University in 2023 to help establish mitochondrial donation in Australia, following the passage of Maeve’s Law the previous year, meaning Australians will be able to benefit directly from what has been learnt in the UK. That same year, the Medical Research Future Fund (MRFF) provided $15 million for the Monash-hosted mitoHOPE Program, to pilot the introduction of mitochondrial donation into Australian clinical practice.
Since late 2023, the Monash team and their partners have been working with the National Health and Medical Research Council (NHMRC) to gain a licence to train embryologists in mitochondrial donation, as a first step towards a clinical trial and clinical application of mitochondrial donation. According to Professor John Carroll, Director of the Monash Biomedicine Discovery Institute and Head of mitoHOPE, the UK studies should give Australia momentum to follow suit.
“We hope to soon obtain our first licence from the NHMRC’s Embryo Research Licensing Committee, so that we can begin training our IVF embryologists in the procedure using donated eggs,” Carroll said.
“The birth of eight babies with a greatly reduced risk of developing mitochondrial DNA disease provides hope for those waiting to access mitochondrial donation and shines new light on the path toward introducing mitochondrial donation in Australia. The mitoHOPE team aims to start recruiting into the clinical trial in about a year, but this is dependent on us gaining the necessary licences.”
Herbert said the UK results were a long time in the making, and that she hopes the successful outcomes will help in navigating Australia’s “rather complex regulatory system”.
“I look forward to continuing this work at Monash University and I hope that the new treatment can soon become available to Australian families affected by these devastating diseases,” she concluded.
How a tiny worm changed a decade of scientific thinking
A tiny roundworm has helped University of Queensland scientists rethink the way sensory nerve...
A scientist, a cooler and a long-haul flight in the fight against MND
Early in January 2026, neuroscientist Dr Rachael Dunlop boarded a flight from Sydney to the US...
Mini lung organoids could help test new treatments
Scientists have developed a simple method for automated the manufacturing of lung organoids...



