Europe agrees to blow the green whistle
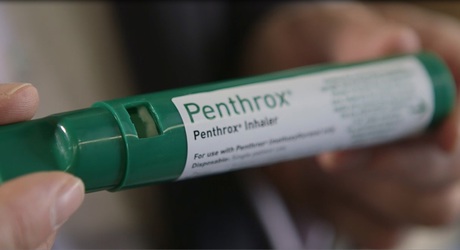
The Australian-invented painkiller Penthrox - commonly known as the ‘green whistle’ - has received initial regulatory approval for sale in the UK and Europe. The milestone has seen manufacturer Medical Developments International (ASX:MVP) team up with CSIRO to increase production of Penthrox’s underlying drug, methoxyflurane, in order to meet this large-scale demand.
MVP CEO John Sharman explained that Penthrox is an inhaled analgesic which has been used by Australian “hospitals, ambulances, defence forces, national sporting leagues, lifesavers and other emergency services” for more than 30 years. Unlike analgesics such as nitrous oxide and morphine, the product provides rapid, strong pain relief whilst also being self-administered, non-addictive, non-narcotic and safe to use.
The device should be available for sale in the UK, Ireland, France and Belgium within the next 30 days. Sharman described the product’s approval in these countries as “the most significant event in the company’s history”.
Sharman also acknowledged CSIRO’s participation in MVP’s new Penthrox manufacturing program, which will enable the company to “upscale our output by a factor of 10 and have very significant cost reductions at the same time”. He said the new manufacturing process has contributed to MVP’s ability to go global.
Medical Developments International (ASX:MVP) shares closed 12.68% higher at $2.51 following the approval announcement on Wednesday. Shares were trading 3.26% higher at $2.53 as of around 3.30 pm on Friday.
A scientist, a cooler and a long-haul flight in the fight against MND
Early in January 2026, neuroscientist Dr Rachael Dunlop boarded a flight from Sydney to the US...
Mini lung organoids could help test new treatments
Scientists have developed a simple method for automated the manufacturing of lung organoids...
Clogged 'drains' in the brain an early sign of Alzheimer’s
'Drains' in the brain, responsible for clearing toxic waste in the organ, tend to get...



