Newly designed drug compound to stop malaria in its tracks
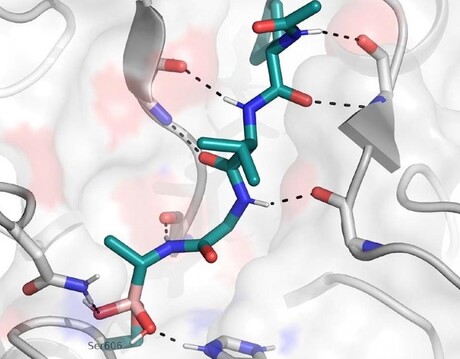
Researchers at The Francis Crick Institute and the Latvian Institute of Organic Synthesis have designed a drug-like compound which effectively blocks a critical step in the malaria parasite’s life cycle, and are now working to develop this compound into a potential first-of-its-kind malaria treatment.
While drugs and mosquito control have reduced levels of malaria over recent decades, the parasite still kills over 400,000 people every year, infecting many more. It has also developed resistance to many existing antimalarial drugs, meaning new treatments that work in different ways are urgently needed.
The UK and Latvian scientists developed a set of compounds designed to stop the parasite being able to burst out of red blood cells — a process vital to its replication and life cycle. They found one compound in particular was highly effective in human cell tests, with their results published in the journal PNAS.
“Malaria parasites invade red blood cells where they replicate many times, before bursting out into the bloodstream to repeat the process,” explained Mike Blackman, lead author and group leader of the Malaria Biochemistry Laboratory at the Crick. “It’s this cycle and build-up of infected red blood cells which causes the symptoms and sometimes fatal effects of the disease.
“If we can effectively trap malaria in the cell by blocking the parasite’s exit route, we could stop the disease in its tracks and halt its devastating cycle of invading cells.”
The new compound works by blocking an enzyme called SUB1, which is critical for malaria to burst out of red blood cells. Existing antimalarials work by killing the parasite within the cell, so the researchers hope this alternative drug action will overcome the resistance the parasite has acquired. Importantly, the compound is also able to pass through the membranes of the red blood cell and of the compartment within the cell where the parasites reside.
The team is continuing to optimise the compound, making it smaller and more potent. If successful, it will need to be tested in further experiments and in animal and human trials to show it is safe and effective, before being made available to people.
“Many existing antimalarial drugs are plant derived and, while they’re incredibly effective, we don’t know the precise mechanisms behind how they work,” noted study author Chrislaine Withers-Martinez, a researcher in the Malaria Biochemistry Laboratory. “Our decades of research have helped us identify and understand pathways crucial to the malaria life cycle, allowing us to rationally design new drug compounds based on the structure and mechanism of critical enzymes like SUB1.
“This approach, which has already been highly successful at finding new treatments for diseases including HIV and hepatitis C, could be key to sustained and effective malaria control for many years to come.”
Please follow us and share on Twitter and Facebook. You can also subscribe for FREE to our weekly newsletters and bimonthly magazine.
Portable point-of-care test detects four common STIs in under an hour
Australian researchers have developed a portable point-of-care test that detects four common...
AusBiotech and Proto Axiom partner on investor-focused life sciences programs
AusBiotech and Proto Axiom have announced a partnership to strengthen national coordination...
The University of Sydney formalises cervical cancer elimination partnership
The success of a cervical cancer elimination program has led to the signing of a memorandum of...




