Organ preservation device is not chopped liver
Organ transplantation currently depends on preserving donor organs by putting them ‘on ice’ - cooling them to slow their metabolism. But this often leads to organs becoming damaged.
Now, in a world first, a donated human liver has been ‘kept alive’ outside a human being and then successfully transplanted into a patient in need of a new liver. The procedure has been performed on two patients on the liver transplant waiting list and both are making excellent recoveries.
The technology was developed at Oxford University and is now being trialled at the liver transplant centre at King’s College Hospital. A donated human liver connected to the device is raised to body temperature and oxygenated red blood cells are circulated through its capillaries. Once on the machine, a liver functions normally just as it would inside a human body.
The results from the first two transplants suggest that the device could be useful for all patients needing liver transplants. Based on preclinical data, the device could also enable the preservation of livers which would otherwise be discarded as unfit for transplantation - potentially as much as doubling the number of organs available for transplant and prolonging the maximum period of organ preservation to 24 hours.
“These first clinical cases confirm that we can support human livers outside the body, keep them alive and functioning on our machine and then, hours later, successfully transplant them into a patient,” said Professor Constantin Coussios of Oxford University’s Department of Engineering Science, one of the machine’s inventors and technical director of OrganOx, the university spin-out created to bring the device from bench to bedside.
“The device is the very first completely automated liver perfusion device of its kind: the organ is perfused with oxygenated red blood cells at normal body temperature, just as it would be inside the body, and can, for example, be observed making bile, which makes it an extraordinary feat of engineering.
“It was astounding to see an initially cold grey liver flushing with colour once hooked up to our machine and performing as it would within the body. What was even more amazing was to see the same liver transplanted into a patient who is now walking around.”
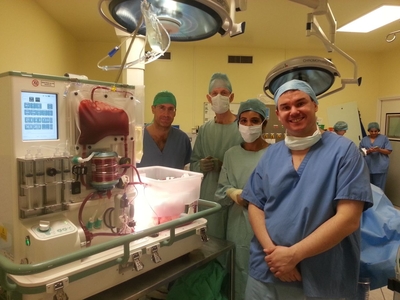
Wayel Jassem, Consultant Liver Transplant Surgeon at King’s College Hospital, who performed both transplant operations, said: “There is always huge pressure to get a donated liver to the right person within a very short space of time. For the first time, we now have a device that is designed specifically to give us extra time to test the liver, to help maximise the chances of the recipient having a successful outcome. This technology has the potential to be hugely significant and could make more livers available for transplant, and in turn save lives.”
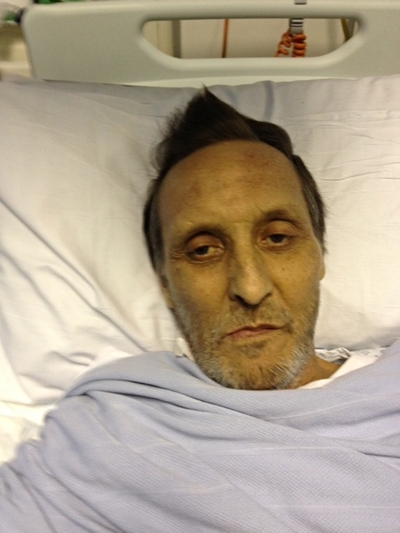
Ian Christie, 62, was the first person to receive a transplanted liver kept alive on the device. In May 2012 he was told he had cirrhosis of the liver and without a transplant he had an estimated 12-18 months to live. He was also told that he would spend 12-18 months on the transplant waiting list.
“I took part in the trial because I just think it’s the right thing to do,” he said. “If the device can help more people in my situation in the future, it’s my duty to help. I trusted that the doctors wouldn’t go ahead with it unless they were absolutely sure so I knew there wasn’t a risk to my transplant.
“I feel better than I’ve felt for 10-15 years, even allowing for the pain and wound that’s got to heal. I’m getting better and better day by day. I just feel so alive!”

Professor Coussios and colleagues, including Professor Peter Friend of Oxford’s Nuffield Department of Surgical Sciences, have been researching the technology since 1994. Professor Friend said: “Transplant surgery is a victim of its own success, with far more people needing transplants than there are donor organs available. This device has the potential to change that situation radically.
“By enabling us to transplant many organs that are unusable with current techniques, this technology could bring benefit to a large number of patients awaiting transplants, many of whom currently die whilst still waiting.
“At present, organ transplantation depends upon cooling the organ to ice temperature to slow down its metabolism, but this does not stop it deteriorating and, if the organ is already damaged in some way, perhaps by being deprived of oxygen, then the combined effect can be disastrous. Many potential donor organs are declined as being unsuitable for this reason.
“This new technique allows us to assess how well an organ is working before having to decide whether to commit a patient to the operation. So this technology promises to quality-assure organs which would otherwise be discarded. This would increase the number of transplants without increasing the risks.”
In 2008, the spin-out company OrganOx was formed, through the university’s technology transfer firm Isis Innovation, in order to commercialise the Oxford research. OrganOx developed the device for these first clinical trials.
OrganOx CEO Dr Les Russell noted that in Europe and the US there are around 13,000 liver transplants undertaken each year out of a waiting list of 30,000 patients. Up to 25% of these patients die while awaiting transplantation, while over 2000 livers are discarded due to damage.
European Space Agency inaugurates deep space antenna in WA
The ESA has expanded its capability to communicate with scientific, exploration and space safety...
Black hole collision supports Hawking's landmark theory
Astrophysicists have witnessed a collision between two black holes that was so loud, they were...
Uncovering differences in wild and domesticated crops
Researchers have revealed insights into the genetic make-up of wild varieties of common crops...



