Breakthrough in imaging biomolecules inside cells
One particular challenge for biologists is seeing all the molecules in an intact tissue sample, down to the level of single cells, simultaneously. Now US researchers have found a way to expand tissue samples so that hundreds of biomolecules can be seen in their native locations, publishing their work in the journal Nature Methods.
Detecting the location of hundreds or thousands of biomolecules — from lipids to metabolites to proteins — in their native environment allows researchers to better understand their functions and interactions. But while many imaging methods provide a view of molecules inside cells, they can track only a select handful of molecules at one time, and they can’t detect all types of biomolecules, including some lipids. Other methods, like regular mass spectrometry, can detect hundreds of molecules but don’t work on intact samples, so researchers can’t see how the biomolecules are oriented.
One promising technique — mass spectrometry imaging — overcomes some of these challenges, as it allows researchers to see hundreds of molecules at one time in intact tissues. However, it doesn’t have high enough resolution to allow detection at the single-cell level. This was the problem being faced by Meng Wang and her team at Howard Hughes Medical Institute’s Janelia Research Campus.
Wang and her team study the fundamental mechanisms behind aging and longevity, and they wanted to detect many different biomolecules in intact tissues to understand how the components change as tissues age. As explained by Wang, “Knowing at each specific location what molecules are there and what is in the neighbouring cells is very important for any kind of biological question.”
It just so happened that Wang’s lab was located down the hall from Janelia Principal Scientist Paul Tillberg, who co-invented a technique called expansion microscopy as a graduate student at MIT. The method uses a swellable hydrogel material to expand samples uniformly in all directions to a point where fine details, like sub-organelle structure, can be detected with a conventional microscope.
Now a decade old, the expansion process is being applied to other methods outside traditional microscopy. Wang, Tillberg and their collaborators at Janelia and the University of Wisconsin–Madison wanted to see if they could use expansion to overcome mass spectrometry imaging’s spatial resolution problem.
The result is the development of a so-called tissue-expansion method compatible with matrix-assisted laser desorption/ionisation mass-spectrometry imaging (TEMI) — a technique that expands tissue samples gradually without having to degrade them at the molecular level, as happens in the original expansion process. By expanding the intact samples in all directions, researchers can use mass spectrometry imaging to simultaneously detect hundreds of molecules at the single-cell level in their native locations.
“This lets you have an untargeted look in the molecular space, and we are trying to bring it closer to what microscopy can do in terms of spatial resolution,” Tillberg said.
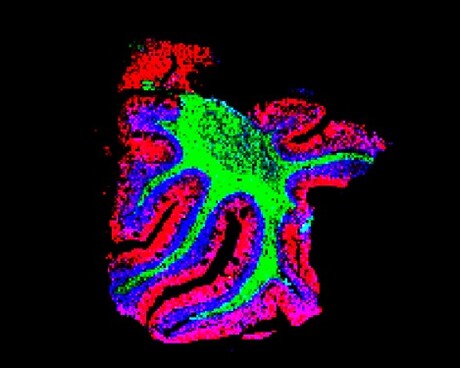
The team used the new technique to delineate the specific spatial patterns of small molecules in different layers of the cerebellum. They found that these molecules — including lipids, peptides, proteins, metabolites and glycans — are not uniformly distributed, as previously thought. Moreover, they found that each specific layer of the cerebellum has its own signature of lipids, metabolites and proteins.
The team was also able to detect biomolecules in kidney, pancreas and tumour tissues, demonstrating that the method can be adapted for many different tissue types. In tumour tissues, they were able to visualise large variations in biomolecules, which could be useful for understanding the molecular mechanisms of tumours and potentially aid in drug development.
“When you can see these biomolecules, then you can start to understand why they have such patterns and how that is related to function,” Wang said. She believes the new technology will allow researchers to track these patterns during development, aging and disease to understand how different molecules contribute to these processes.
Because the new method doesn’t require adding hardware to an existing mass spectrometry imaging system, and the expansion technique is relatively easy to learn, the researchers hope it will be used by many labs around the world. They also hope the new technique will make mass spectrometry imaging a more useful tool for biologists, and have laid out a detailed description of the new method and a roadmap for adapting it to other tissue types.
“We wanted to develop something that did not require specialised instruments or procedures, but can be broadly adopted,” Wang said.
From assistance to agency in biopharma R&D: benefits of next-gen AI-native lab notebooks
The next generation of AI-native lab notebooks has the potential to turn AI into an active...
Images reveal "incredible" detail of industrial micro-CT scanner
Images of intricate biological structures and everyday items have revealed the capabilities of a...
MRI scanner to advance medical breakthroughs at Monash
Siemens Healthineers' MAGNETOM Cima.X 3T is claimed to be Victoria's most advanced,...



