Copper zeolite converts methane to methanol
A newly developed bio-inspired zeolite may pave the way to small-scale ‘gas-to-liquid’ technologies which convert natural gas to fuels and starting materials for the chemical industry. The catalyst has been developed by European researchers, who published their breakthrough in the journal Nature Communications.
In an era of depleting mineral oil resources, natural gas is becoming ever more relevant; however, the gas is also difficult to transport and not easily integrated in the existing industrial infrastructure. This can be solved by gas-to-liquid technologies, which convert methane (the principal component of natural gas) to synthesis gas, from which liquid methanol and hydrocarbons are produced. This approach, however, is only feasible at very large scales.
Of all the conceptually promising smaller scale processes for the direct conversion of methane, the partial oxidation to methanol seems the most viable since it allows for lower operating temperatures, making it more inherently safe and energy efficient. Researchers from the University of Amsterdam, Eindhoven University of Technology and Technische Universität München (TUM) are currently focusing on a bio-inspired method enabling such partial methane oxidation.
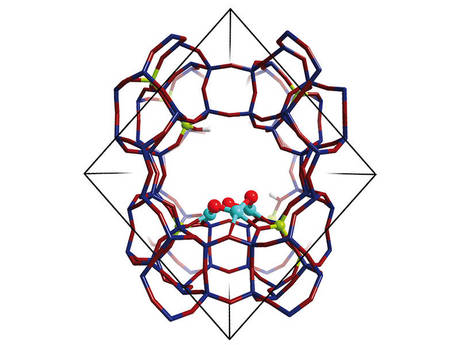
The team have been developing a modified zeolite, a highly structured porous material, developed at TUM by a research group led by Professor Johannes Lercher. This copper-exchanged zeolite, with a mordenite structure, mimics the reactivity of an enzyme known to efficiently and selectively oxidise methane to methanol. The researchers have now provided a detailed molecular insight into the way the zeolite mimics the active site of the enzyme methane monooxygenase (MMO).
The team showed that the micropores of the zeolite provide a perfect confined environment for the highly selective stabilisation of an intermediate copper-containing trimer molecule. Trinuclear copper-oxo clusters were identified that exhibit a high reactivity towards activation of carbon-hydrogen bonds in methane and its subsequent transformation to methanol.
“The developed zeolite is one of the few examples of a catalyst with well-defined active sites evenly distributed in the zeolite framework — a truly single-site heterogeneous catalyst,” said Professor Lercher. “This allows for much higher efficiencies in conversion of methane to methanol than with zeolite catalysts previously reported.”
The research also showed the unequivocal linking of the structure of the active sites with their catalytic activity. This renders the zeolite a promising material in achieving levels of catalytic activity and selectivity comparable to enzymatic systems.
A non-destructive way to locate microplastics in body tissue
Currently available analytical methods either destroy tissue in the body or do not allow...
Rapid imaging method shows how medicine moves beneath the skin
Researchers have developed a rapid imaging technique that allows them to visualise, within...
Fluorescent molecules glow in water, enhancing cell imaging
Researchers have developed a new family of fluorescent molecules that glow in a surprising way,...



