The many faces of viral research
It is sometimes said that the best ideas are those already thought of, and that is exactly what structural biologist Dr Fasséli Coulibaly in Melbourne is banking on with his research on viruses. By deciphering viral protein structures that have evolved over thousands of years, he hopes to stop viral infections in their tracks and improve delivery of vaccines against a variety of diseases to the developing world.
ARC Future Fellow, Dr Fasséli Coulibaly heads the Structural Virology Laboratory at Monash University’s School of Biomedical Sciences in Melbourne. After arriving in Australia (from France via New Zealand) as a one-man band in 2007, he now runs a diverse research program centred on viruses, particularly the protein structures that make these little pathogenic packages so efficient, and so elusive. The systems he studies range from fascinating insect viruses that encage themselves in Zen-like crystals for surviving the lean times, to human pathogens such as the poxvirus family that includes the notorious smallpox virus.
“We study these different systems for various reasons. One of the drivers is to work out, at the protein structure level, exactly how viruses assemble and interact with their cellular hosts and then use that knowledge to better fight these viruses.”
But probably the major driver of Coulibaly’s career-long fascination with viruses lies in the enormous diversity and extraordinary functional efficiency of viruses.
“Knowing more about these aspects of viruses can tell us lots about how proteins in general work across the biological spectrum. Primarily we are looking at every step of the viral infection - assembly, replication, virulence - across a variety of projects.”
Two current thrusts in the lab are Coulibaly’s poxvirus work and their venture into the world of vaccine delivery using viral microcrystals … with both poised to have far-reaching benefits.
Contributing to a long history
Poxviruses have long been used as model systems in viral research because of their complex infectious cycle and their remarkable ability to hijack and neutralise cellular defence mechanisms.
Indeed, the eradication of smallpox by the end of the 1970s was the culmination of a worldwide vaccine effort against poxvirus infections.
As Coulibaly emphasised, “Pox is an ‘old’ disease in humans that has had a huge impact on history. Smallpox reportedly claimed the life of Egyptian pharaoh Ramses V around 1150 BC and 3000 years later was blamed for the fall of the mighty Aztec civilisation when the newly introduced smallpox virus wiped out over 3 million people.
“Even today, this family of viruses is still considered a threat to health through existing diseases such as the skin disease Molluscum contagiosum, smallpox-like diseases such as monkeypox that can jump from monkeys to humans … and of course there is always the bioterrorism potential of deliberate smallpox release - a very small risk but the impact would be massively devastating.
“Poxviruses are also fascinating because they are the largest and most complex viruses that infect humans, and they are still teaching us a lot about the cells that they infect and the many systems that viruses use to circumvent the host defences.”
In recent work on the prototype mammalian poxvirus Vaccinia (famous as the active constituent of the smallpox vaccine), Coulibaly and Alok Mitra, from the University of Auckland, used a combination of X-ray crystallography and electron microscopy to solve the structure of a Vaccinia scaffolding protein called D13 and confirm its role in assembling the immature poxvirus particles necessary for infection.

Coulibaly is hoping they have found the Achilles heel of the virus.
“At this early stage, the virus has to modify the membranes of the host cells to make its own articles, and it turns out that if we delete or neutralise this single protein, the virus dies before any infectious particle can be formed and infection is therefore blocked. D13 is also a target of the antibiotic rifampicin, and so we are now looking to make small-molecule inhibitors of poxvirus assembly.”
Good things do go into small packages
Another major part of Coulibaly’s work at Monash is a vaccine delivery project, which is based on a single protein produced, in this case, by insect viruses.
This exciting research venture is funded by the Bill & Melinda Gates Foundation and is a collaboration with Rosemary French at the Burnet Institute and Lorena Brown at University of Melbourne.
“Insect viruses are very special compared to all mammalian viruses, because they don’t infect as free virus particles,” explained Coulibaly. “Instead, they actually embed themselves into stable, crystalline matrices inside the infected cells, called polyhedra, that the virus itself makes by overexpressing one of its proteins, appropriately named polyhedrin.”
These relatively massive structures (up to several microns across), sometimes containing up to 1000 virus particles, have evolved to protect the free viruses from dehydration (and almost anything else) once the infected insect dies and the cells rupture.
Then, when another insect larva comes along and eats the crystal, the matrix dissolves in the alkaline pH of the gut, freeing all the virus particles to go on their merry infecting way, make more crystals and so on. A very nifty process indeed, and one that sparked Coulibaly’s interest as a potential microparticle carrier system for all sorts of stuff including fluorescent probes, growth factors, immunogenic proteins and drugs.
The power of crystals
The polyhedra and micropackages idea had been rattling around in Coulibaly’s head long before he successfully secured the initial Phase I Gates funding in 2009. In fact, it started a few years before at the University of Auckland where Coulibaly was doing postdoctoral studies on poxvirus assembly, and fellow structural biologist Peter Metcalf threw him a tantalising protein crystallography challenge to think about in his spare time.
“Peter was trying to determine the structure of one of these infectious viral crystals from a cypovirus that infects silkworm,” said Coulibaly.
Although first viewed by electron microscopy in the late 1940s, virus polyhedra have remained largely uncharacterised in terms of atomic structure because of their extremely small size and the technical limits of X-ray crystallography.
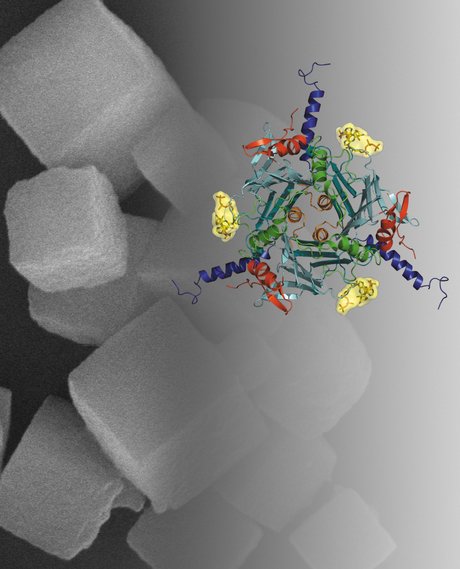
Clearly one not to back away from such a challenge, Coulibaly went to work with Metcalf and, in 2007, determined the first atomic structure of polyhedra purified directly from infected insects. The work was published in Nature and represented the smallest crystals used for solving a new protein structure by X-ray crystallography.
“Importantly, our results also suggested molecular bases for the outstanding stability of polyhedra against most chemical treatments (SDS, urea, acids) and their rapid dissolution at the alkaline pH encountered in the mid-guts of insect larvae.”
Two years later at Monash University, Coulibaly published the second structure of a polyhedra determined by X-ray crystallography from microcrystals produced in vivo, this time from the Baculovirus family of insect viruses. This study revealed interesting and unexpected differences in the polyhedra building blocks from the two viral species that suggested novel information about viral evolution - also a subject of intense interest for Coulibaly.
How they did it - the techie bits
“There were several reasons at that time for Nature accepting this work, but in part it was because of the major technical achievement needed to solve crystals that small by X-ray crystallography. And that approach is still used now to try and solve these sorts of structures more efficiently,” Coulibaly said.
The natural stability and abundant availability of the microcrystals helped, but basically the team had to develop a number of strategies from scratch along the way, even just to handle them for analysis. They used specifically fabricated micromatrices as holders for the crystals to enable separation and alignment of the crystals for data collection in the diffractometer.
“There were also other hardware developments in X-ray diffraction just becoming available at that time, and specifically the third-generation synchrotron microfocus beamlines that are especially designed to transmit X-rays through these tiny crystals,” explained Coulibaly.
“So, doing all of this and using the X06SA beamline at the Swiss Light Source Synchrotron near Zurich (and later at the Australian Synchrotron), we were able to observe single-crystal diffraction. From there it was just lots of time and hard work to analyse several thousand of these microcrystals harvested from insect cells - and about 2 years later we had our first atomic structure of the cypovirus polyhedra to a resolution of 2 angstrom.”
More than just pretty faces
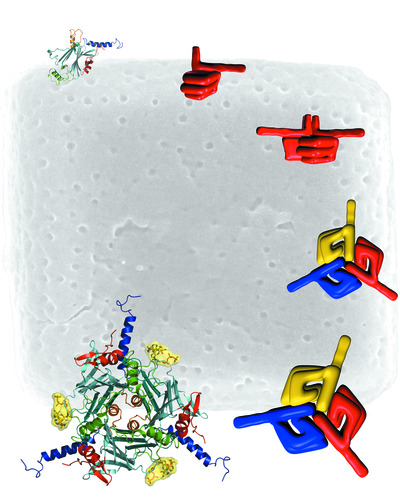
Subsequent studies showed that the polyhedra could also be engineered using well-established insect-cell-expression systems to express other small molecules such as drugs or fluorescent probes, without losing their robust nature. And, unlike existing virus-like nanoparticles, the fine-crystal packages custom made by viruses are easy to manipulate because of their size and strength, and can accommodate a wide range of cargoes including growth factors and full-length protein antigens.
Indeed, it seems that size really doesn’t matter when it comes to packing the polyhedra with useful molecules such as HIV antigens.
“One of the key features that we immediately saw in these structures when thinking about them as a carrier platform for vaccines was their size - they can hold a huge amount of protein inside them, so something like an entire antigen should not be a problem. And so far we haven’t reached a limit in terms of size. We can use native full-length antigens and they are released exactly as the immune cells would normally see them in vivo,” Coulibaly said.
And best of all, the polyhedral carriers are all produced in vivo, in insect cell culture, where good quality control can be maintained over the production process. Plus, the isolation process from the cells has no need for complex protein purification or formulation, avoiding problems such as the expressed protein becoming insoluble or damaged during purification.
Capitalising on the Grand Challenge
Coulibaly is keen to emphasise that the vaccine project is a separate focus in his lab and was only made possible due to the Gates Foundation funding.
“When I first came to Monash, I focused solely on the structural biology of viruses, which is my area of expertise. But I always had this plan, and so Rosemary Ffrench and I applied for Phase I Grand Challenges Exploration funding from the Bill & Melinda Gates Foundation to design ultrastable microcapsules for HIV and flu vaccination based on viral polyhedra.”
The team’s success in gaining that funding allowed Coulibaly to take this new direction, which is exactly the aim of the Gates scheme - to attract and support translational aspects of basic research from people across different fields.
“And, now we have been very lucky to secure Phase II funding to continue the vaccine work (see box), which was quite unexpected but very welcome. Extensions are rare and the larger grants are highly competitive. That started about 10 months ago and is very exciting for us.”
So, besides being a great tale of good science, brains, hard work, innovation and, of course, some luck, Fasséli Coulibaly’s research story highlights yet again the importance of funding basic science as the best way to generate novel discoveries with potentially far-reaching health consequences.
***************************************************
MicroCubes as vaccines for the developing world
The Bill and Melinda Gates Grand Challenges Explorations (GCE) funding scheme invests in the early stages of bold ideas with real potential to solve the problems people in the developing world face every day.
The Phase I funding awarded to Coulibaly’s team in 2009 enabled proof-of-concept studies to establish MicroCubes as a promising platform to deliver new and more potent vaccines with increased stability, obviating the need for refrigeration and extending the vaccine applications for use in the developing world. These virus polyhedra-based microcarriers were successfully engineered to accommodate various antigens, including an HIV protein, and to elicit strong T-cell responses. Their unique crystalline organisation results in slow release of the antigen and stimulates both arms of the immune system in mice.
The highly competitive Phase II GCE grants recognise ideas that have made significant progress towards implementation, and Coulibaly’s project is one of only four awardees announced last year.
“In the Phase II work, we will assess the suitability of MicroCubes as a generic vaccine platform, while working on a candidate flu vaccine. The fantastic tools available for research on influenza virus should facilitate translating the preclinical studies to humans,” Coulibaly said.
“We’re hoping to establish … that MicroCubes have unique properties that also warrant their development as a vaccine vector targeting infectious diseases with the highest burden in developing countries - malaria, TB and HIV.”
***************************************************

A scientist, a cooler and a long-haul flight in the fight against MND
Early in January 2026, neuroscientist Dr Rachael Dunlop boarded a flight from Sydney to the US...
Mini lung organoids could help test new treatments
Scientists have developed a simple method for automated the manufacturing of lung organoids...
Clogged 'drains' in the brain an early sign of Alzheimer’s
'Drains' in the brain, responsible for clearing toxic waste in the organ, tend to get...



