Radio waves improve survival in liver cancer patients
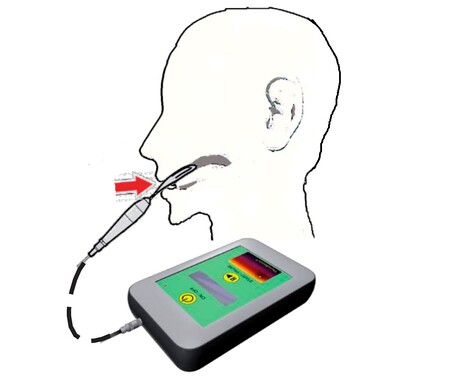
Researchers at Wake Forest School of Medicine have shown that a targeted therapy using non-thermal radio waves is safe to use in the treatment of hepatocellular carcinoma (HCC), the most common type of liver cancer. The team’s findings, published in the journal 4Open, also indicate an improvement in overall survival.
“HCC accounts for nearly 90% of all liver cancers, and current survival rates are between six and 20 months,” said Boris Pasche, Chair of Cancer Biology and Director of the Wake Forest Baptist Comprehensive Cancer Center. “Currently, there are limited treatment options for patients with this advanced liver cancer.”
For the study, researchers used a handheld device called TheraBionic P1, invented by Pasche and Alexandre Barbault of TheraBionic, that works by delivering cancer-specific, amplitude-modulated radiofrequency electromagnetic fields (AM RF EMF) programmed specifically for HCC. The frequencies used are specific to the patient’s type of cancer as identified through tumour biopsies or blood work, with Pasche and Barbault having discovered radio frequencies for 15 different types of cancer back in 2009.
TheraBionic P1 emits radio frequencies via a spoon-shaped antenna, which is placed on the patient’s tongue during treatment and is administered three times a day for one hour to deliver low levels of radiofrequency electromagnetic fields throughout the patient’s body. In previous studies, the device — which received breakthrough designation from the FDA in 2019 — was shown to block the growth of liver cancer cells in the body without damaging healthy cells.
For the current study, 18 patients with advanced HCC participated and received treatment with the device. Researchers also analysed previously published data on 41 patients from a Phase 2 study and historical controls from earlier clinical trials.
“Our findings show an improvement in overall survival of more than 30% in patients with well-preserved liver function and also in those with more severe disease,” Pasche said. The researchers also tracked side effects, and no patients stopped TheraBionic P1 treatment because of adverse reactions.
“We’re encouraged by these initial findings,” Pasche said. “Our study shows a benefit in overall survival, and the treatment isn’t associated with any significant side effects.”
Pasche noted that the study does have several limitations because of the small sample size and “selection bias inherent in the use of historical control data”. However, two additional clinical trials are now underway: one is a single-centre study to assess the safety and effectiveness of the TheraBionic device in combination with Regorafenib, a chemotherapy drug, as a second-line treatment; the other is a multicentre, double-blind, randomised study comparing TheraBionic with a placebo to assess the safety and effectiveness of the device as a third-line therapy in the treatment of advanced HCC.
Please follow us and share on Twitter and Facebook. You can also subscribe for FREE to our weekly newsletters and bimonthly magazine.
Portable point-of-care test detects four common STIs in under an hour
Australian researchers have developed a portable point-of-care test that detects four common...
AusBiotech and Proto Axiom partner on investor-focused life sciences programs
AusBiotech and Proto Axiom have announced a partnership to strengthen national coordination...
The University of Sydney formalises cervical cancer elimination partnership
The success of a cervical cancer elimination program has led to the signing of a memorandum of...




