Nano camera for real-time monitoring of chemical reactions
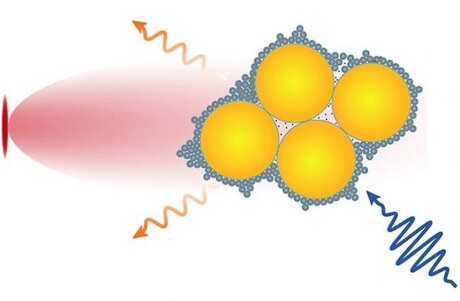
Researchers from the University of Cambridge have made a tiny camera, held together with ‘molecular glue’, that allows them to observe chemical reactions in real time. Their results are reported in the journal Nature Nanotechnology.
Nature controls the assemblies of complex structures at the molecular scale through self-limiting processes. However, mimicking these processes in the lab is usually time-consuming, expensive and reliant on complex procedures.
“In order to develop new materials with superior properties, we often combine different chemical species together to come up with a hybrid material that has the properties we want,” said Professor Oren Scherman, who led the research. “But making these hybrid nanostructures is difficult, and you often end up with uncontrolled growth or materials that are unstable.”
Prof Scherman collaborated with researchers from Cambridge’s Cavendish Laboratory and University College London to develop a method that uses a molecular glue called cucurbituril (CB), which interacts strongly with both semiconductor nanocrystals called quantum dots and gold nanoparticles. The researchers used the small nanocrystals to control the assembly of the larger nanoparticles through a process they coined interfacial self-limiting aggregation. The process leads to permeable and stable hybrid materials that interact with light.
The team added the individual components, along with the molecule they wanted to observe, to water at room temperature. Previously, when gold nanoparticles were mixed with the molecular glue in the absence of quantum dots, the components underwent unlimited aggregation and fell out of solution. However, with the strategy developed by the researchers, quantum dots mediate the assembly of these nanostructures so that the semiconductor–metal hybrids control and limit their own size and shape. In addition, these structures stay stable for weeks.
“This self-limiting property was surprising; it wasn’t anything we expected to see,” said co-author Dr Jade McCune. “We found that the aggregation of one nanoparticulate component could be controlled through the addition of another nanoparticle component.”
The camera harvests light within the semiconductors, inducing electron transfer processes like those that occur in photosynthesis, which can be monitored using the gold nanoparticle sensors and spectroscopic techniques. This enabled the team to observe in real time the formation of radical species — a molecule with an unpaired electron — and products of their assembly such as sigma dimeric viologen species, where two radicals form a reversible carbon–carbon bond. The latter species had been theorised but never observed.
“We were surprised how powerful this new tool is, considering how straightforward it is to assemble,” said first author Dr Kamil Sokołowski.
“This platform is a really big toolbox considering the number of metal and semiconductor building blocks that can be now coupled together using this chemistry — it opens up lots of new possibilities for imaging chemical reactions and sensing through taking snapshots of monitored chemical systems. The simplicity of the set-up means that researchers no longer need complex, expensive methods to get the same results.”
Researchers from the Scherman lab are currently working to further develop these hybrids towards artificial photosynthetic systems and (photo)catalysis where electron-transfer processes can be observed directly in real time. The team is also looking at mechanisms of carbon–carbon bond formation as well as electrode interfaces for battery applications.
“This platform will unlock a wide range of processes, including many materials and chemistries that are important for sustainable technologies,” Prof Scherman said. “The full potential of semiconductor and plasmonic nanocrystals can now be explored, providing an opportunity to simultaneously induce and observe photochemical reactions.”
Please follow us and share on Twitter and Facebook. You can also subscribe for FREE to our weekly newsletters and bimonthly magazine.
From assistance to agency in biopharma R&D: benefits of next-gen AI-native lab notebooks
The next generation of AI-native lab notebooks has the potential to turn AI into an active...
Images reveal "incredible" detail of industrial micro-CT scanner
Images of intricate biological structures and everyday items have revealed the capabilities of a...
MRI scanner to advance medical breakthroughs at Monash
Siemens Healthineers' MAGNETOM Cima.X 3T is claimed to be Victoria's most advanced,...




