World's first patient treated with personalised gene editing
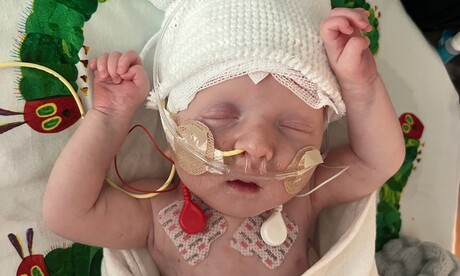
In what is being described as a historic medical breakthrough, an infant diagnosed with a rare genetic disorder has been treated with a customised CRISPR gene editing therapy by a team at the Children’s Hospital of Philadelphia (CHOP) and Penn Medicine.
The story has been detailed in a study published in The New England Journal of Medicine and presented at the American Society of Gene & Cell Therapy’s Annual Meeting in New Orleans. It is believed that the findings could provide a pathway for gene-editing technology to be successfully adapted to treat individuals with rare diseases for whom no medical treatments are available.
The case revolves around an infant known as KJ, who was just two days old when a doctor at the Hospital of the University of Pennsylvania noticed he had become unusually lethargic, he wasn’t eating and he struggled to maintain his temperature. The doctor checked KJ’s blood ammonia level — which can be a marker for some metabolic diseases — and found it was extremely high. After being moved to CHOP for tests and examinations, KJ was deemed to have a urea cycle disorder — a genetic condition caused by deficiencies in specific enzymes that leads to a toxic build-up of ammonia in the body.
During the normal breakdown of proteins in the body, ammonia is naturally produced. Typically, our bodies know to convert the ammonia to urea and then excrete that urea through urination. However, children with a urea cycle disorder lack an enzyme in the liver needed to convert ammonia to urea. Ammonia then builds up to a toxic level, which can cause organ damage, particularly in the brain and the liver. Episodes of increased ammonia can thus put patients at risk for ongoing, lifelong neurologic damage or even prove fatal.
After doctors placed KJ on dialysis to filter the ammonia out of his blood and stabilise his health, they performed genetic tests to figure out exactly what type of urea cycle disorder he had, as determining which gene or enzyme was affected could alter the recommended treatment. They discovered that KJ had been born with a rare metabolic disease known as severe carbamoyl phosphate synthetase 1 (CPS1) deficiency, caused by the CPS1 gene.
The only known lifesaving treatment for CPS1 deficiency was a liver transplant, which is often better suited to children who are older, larger and in better health; treatments for younger, smaller patients are sadly lacking. Nonetheless, KJ was added to the National Transplant Waiting List with the hope he would be stable enough for a liver transplant when — or if — a suitable organ became available. In the meantime, KJ would spend the first several months of his life at CHOP, on a very restrictive diet, where he could receive constant support and monitoring around the clock.
Developing a custom treatment
Before KJ was even born, a team of scientists at CHOP and the University of Pennsylvania were researching how to use gene editing to create customised treatments for diseases like CPS1 deficiency. Leading the group were Dr Rebecca Ahrens-Nicklas, a paediatric geneticist and Director of CHOP’s Gene Therapy for Inherited Metabolic Disorders Program, and Dr Kiran Musunuru, a cardiologist, geneticist and gene editor at Penn Medicine.
Ahrens-Nicklas and Musunuru first began collaborating to study the feasibility of creating customised gene editing therapies for individual patients in 2023, building upon many years of research into rare metabolic disorders, as well as the feasibility of gene editing to treat patients. CRISPR-based gene editing can already be used to precisely correct disease-causing variants in the human genome, but up until this point, gene editing tools have been built to target more common diseases that affect tens or hundreds of thousands of patients, such as the two diseases for which there currently are FDA-approved therapies: sickle cell disease and beta thalassemia. Furthermore, relatively few diseases benefit from a ‘one-size-fits-all’ gene editing approach since so many disease-causing variants exist, so many patients with rare genetic diseases have been left behind.
“We’ve been practising developing … personalised therapies for about two years now with the idea that someday we might be in a position where we could very rapidly try to figure out how to use gene editing to correct a patient’s broken gene that’s responsible for their disease,” Musunuru said. During this time, the researchers learned a great deal about ways to correct faulty genes. With each potential genetic change they considered for gene editing therapy, the time it took to find a personalised solution shortened.
After years of preclinical research with similar disease-causing variants, Ahrens-Nicklas and Musunuru targeted KJ’s specific variant of CPS1. They approached KJ’s family about the possibility of participating in a gene-editing research study, and after just six months of research and successful results in the lab, the team had designed and manufactured a base editing therapy delivered via lipid nanoparticles to the liver in order to correct KJ’s faulty enzyme. In essence, base editing allows scientists to rewrite DNA one ‘letter’ at a time.
On 25 February 2025, between six and seven months of age, KJ received his first infusion of this experimental therapy, making him the first patient ever to receive a systemic personalised gene editing drug. The drug was delivered in an IV line and flowed into the bloodstream, travelling to the liver. KJ received the lowest possible dose of the therapy to allow his body time to adapt and minimise any risks.
“CRISPR, a gene editor, enters the nucleus of the cell,” Musunuru explained. “In this case, we programmed it to go to the site of the genetic variant that was causing the disease in KJ.”
“The drug was specifically designed for KJ and the genetic variants he has,” Ahrens-Nicklas added. “It’s personalised medicine.”
Just a few days after KJ’s first infusion, he showed signs of improvement. The colour in his cheeks returned; he could tolerate more protein in his diet without causing a toxic increase in ammonia; and clinicians were able to slowly decrease his medication. Over the next two months, KJ received two additional infusions at higher levels. He has not had any serious side effects to date and is now growing and thriving, even recovering from typical childhood illnesses like rhinovirus without ammonia building up in his body.
KJ continues to be monitored as an inpatient at CHOP — indeed, he will need careful monitoring for the rest of his life — and longer follow-up will be needed to fully evaluate the benefits of the therapy. But Ahrens-Nicklas believes the initial findings are quite promising, while KJ’s parents are looking forward to soon welcoming him home.
“Years and years of progress in gene editing and collaboration between researchers and clinicians made this moment possible, and while KJ is just one patient, we hope he is the first of many to benefit from a methodology that can be scaled to fit an individual patient’s needs,” Ahrens-Nicklas said.
“We want each and every patient to have the potential to experience the same results we saw in this first patient, and we hope that other academic investigators will replicate this method for many rare diseases and give many patients a fair shot at living a healthy life,” Musunuru added. “The promise of gene therapy that we’ve heard about for decades is coming to fruition, and it’s going to utterly transform the way we approach medicine.”
Five scenarios for the future of Antarctic life
A team of Australian and international researchers have predicted five possible outcomes for how...
Could this biosensor bypass labs with onsite PFAS detection?
La Trobe University has developed a portable biosensor that may allow rapid, onsite detection of...
How a tiny worm changed a decade of scientific thinking
A tiny roundworm has helped University of Queensland scientists rethink the way sensory nerve...




